In the realm of chemistry, moles and grams represent two fundamental units of measurement. A mole is a unit that quantifies the amount of a substance, while grams measure the mass. The conversion between these units involves utilizing the molar mass of a substance, which is the mass of one mole of that substance. This process is foundational for many calculations in chemistry, from stoichiometry to molecular weight determination. This comprehensive guide provides a detailed exploration of how to convert moles to grams, offering step-by-step instructions, practical examples, and useful tips. Whether you're a student needing to complete homework assignments or a professional looking to refine your skills, this guide will equip you with the knowledge and confidence to perform these conversions with ease.
Table of Contents
1. Understanding Moles 2. Understanding Grams 3. What is Molar Mass? 4. How to Determine Molar Mass? 5. Step-by-Step Guide to Convert Moles to Grams 6. Example Conversions 7. Common Mistakes to Avoid 8. Applications in Chemistry 9. Why is Converting Moles to Grams Important? 10. How Does Temperature Affect Mole to Gram Conversion? 11. Tools for Converting Moles to Grams 12. Advanced Conversion Techniques 13. Frequently Asked Questions 14. External Link 15. Conclusion
Understanding Moles
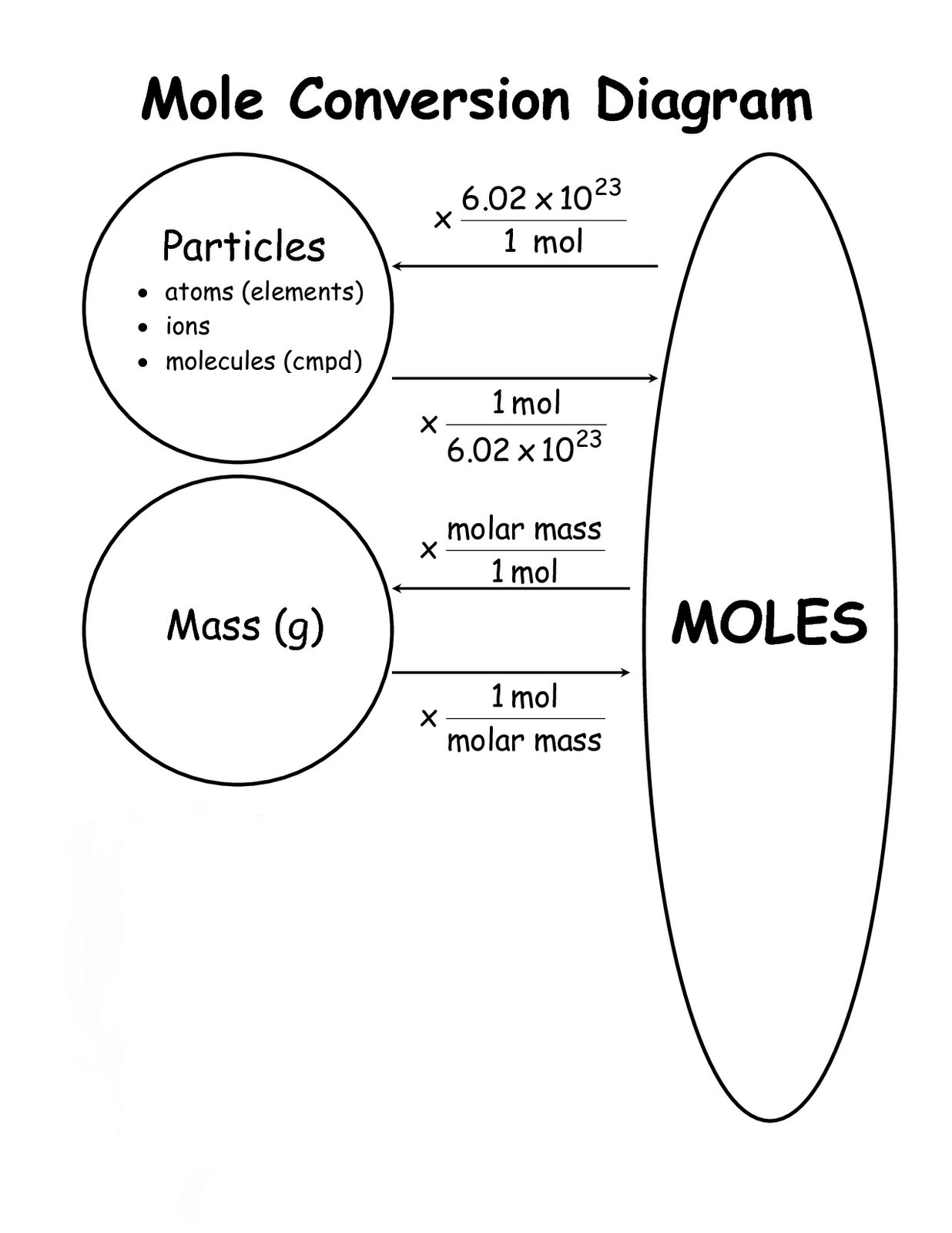
The concept of the mole is central to chemistry, serving as a bridge between the microscopic world of atoms and molecules and the macroscopic world we can observe. The mole is defined as the amount of substance that contains exactly 6.02214076 × 1023 elementary entities, be it atoms, molecules, ions, or other particles. This number is known as Avogadro's number, named after the Italian scientist Amedeo Avogadro.
Read also:Love In The Limelight The Story Of Peso Plumas Girlfriend
The mole allows chemists to count particles in a practical way, as counting individual atoms or molecules would be impractical due to their incredibly small size. Instead, by using moles, we can relate the mass of a substance to the number of particles it contains. This relationship is essential for understanding chemical reactions and stoichiometry, as it allows us to quantify the reactants and products involved.
For instance, when a chemical equation is balanced, it often describes the reaction in terms of moles. This means that the coefficients in a balanced equation can be interpreted as the number of moles of each substance involved. Understanding moles is therefore critical for predicting the amounts of products formed in a reaction, or the amount of reactants required.
Understanding Grams
Grams are a unit of mass in the metric system, widely used in scientific contexts. In chemistry, grams are used to measure the mass of substances, providing a tangible way to relate the quantity of a substance to its physical properties. The use of grams allows for easy comparison of the masses of different substances, facilitating calculations and experiments.
When converting moles to grams, the molar mass of a substance is used. Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a given substance. It is calculated by summing the atomic masses of all the atoms in a molecule, as listed in the periodic table. This allows us to convert between the number of moles and the mass of a substance, making grams an indispensable unit in chemistry.
The use of grams extends beyond chemistry, as it is a fundamental unit in various scientific and engineering fields. Understanding the role of grams in conversion processes is key to accurately measuring and handling chemicals, ensuring precise results in experiments and industrial applications.
What is Molar Mass?
Molar mass is a crucial concept in chemistry, representing the mass of one mole of a given substance. It is expressed in grams per mole (g/mol) and is used to convert between the number of moles and the mass of a substance. The molar mass of an element is typically found in the periodic table, where it is listed as the atomic mass in atomic mass units (amu). For compounds, the molar mass is calculated by summing the atomic masses of all the constituent atoms.
Read also:Deborah Norville Age An Insight Into Her Life And Career
To calculate the molar mass of a compound, one must first identify the chemical formula and the atomic masses of each element involved. For example, to find the molar mass of water (H2O), we use the atomic masses of hydrogen (1.01 g/mol) and oxygen (16.00 g/mol):
- 2 × 1.01 g/mol (for two hydrogen atoms) = 2.02 g/mol
- 1 × 16.00 g/mol (for one oxygen atom) = 16.00 g/mol
- Total molar mass of H2O = 18.02 g/mol
Understanding molar mass is essential for converting moles to grams, as it provides the necessary conversion factor. By using molar mass, we can accurately calculate the mass of a substance needed for a reaction or produced as a result.
How to Determine Molar Mass?
Determining the molar mass of a substance is a straightforward process that involves using the periodic table to find the atomic masses of the elements involved. Here is a step-by-step guide to calculating molar mass:
- Identify the chemical formula: Determine the chemical formula of the substance for which you want to calculate the molar mass. This will indicate the number of each type of atom present in the compound.
- Find atomic masses: Use the periodic table to find the atomic mass of each element in the compound. Atomic masses are typically listed in atomic mass units (amu), which are equivalent to grams per mole (g/mol).
- Multiply by the number of atoms: For each element, multiply its atomic mass by the number of atoms of that element present in the compound. This calculates the contribution of each element to the overall molar mass.
- Sum the contributions: Add the contributions of all elements together to obtain the total molar mass of the compound.
By following these steps, you can accurately determine the molar mass of any compound, allowing for precise conversion between moles and grams.
Step-by-Step Guide to Convert Moles to Grams
Converting moles to grams is a fundamental conversion in chemistry, essential for calculating the mass of a substance given its amount in moles. Here is a detailed step-by-step guide to performing this conversion:
- Determine the number of moles: Identify the amount of substance in moles that you wish to convert to grams. This information may be provided in a problem statement or determined experimentally.
- Find the molar mass: Calculate or look up the molar mass of the substance. This can be done by summing the atomic masses of all the atoms in the molecule, as described in the previous section.
- Use the conversion formula: The conversion from moles to grams is achieved using the formula:
Mass (grams) = Number of moles × Molar mass (g/mol) - Calculate the mass: Multiply the number of moles by the molar mass to obtain the mass of the substance in grams.
By following these steps, you can accurately convert moles to grams, ensuring precise measurements for chemical reactions and experiments.
Example Conversions
To further illustrate the process of converting moles to grams, let's explore a few example conversions:
Example 1: Converting Moles of Water to Grams
Suppose you have 0.5 moles of water (H2O) and wish to convert this to grams. The molar mass of water is 18.02 g/mol, as calculated earlier.
- Mass (grams) = 0.5 moles × 18.02 g/mol = 9.01 grams
Therefore, 0.5 moles of water is equivalent to 9.01 grams.
Example 2: Converting Moles of Sodium Chloride to Grams
Consider 2 moles of sodium chloride (NaCl), a common table salt. The molar mass of NaCl is 58.44 g/mol (23.00 g/mol for sodium and 35.44 g/mol for chlorine).
- Mass (grams) = 2 moles × 58.44 g/mol = 116.88 grams
Thus, 2 moles of sodium chloride correspond to 116.88 grams.
Example 3: Converting Moles of Glucose to Grams
If you have 1 mole of glucose (C6H12O6), you can convert it to grams using its molar mass, which is 180.18 g/mol.
- Mass (grams) = 1 mole × 180.18 g/mol = 180.18 grams
Therefore, 1 mole of glucose equals 180.18 grams.
These examples demonstrate the simplicity and accuracy of converting moles to grams, a crucial skill in chemistry.
Common Mistakes to Avoid
When converting moles to grams, there are several common mistakes that can lead to errors in calculations. By being aware of these pitfalls, you can ensure more accurate results:
- Incorrect molar mass: Failing to accurately determine the molar mass of a substance can result in significant errors. Always double-check calculations and verify atomic masses using a reliable periodic table.
- Units mismatch: Ensure that the units used in calculations are consistent. Molar mass should be in grams per mole (g/mol), and the final mass should be in grams.
- Rounding errors: While rounding can simplify calculations, excessive rounding can lead to inaccuracies. Maintain precision by using appropriate significant figures.
- Overlooking significant figures: Pay attention to significant figures in both the given data and final results, as they reflect the precision of measurements and calculations.
- Misinterpretation of chemical formulas: Ensure correct interpretation of chemical formulas, as errors in counting atoms can affect molar mass calculations.
By avoiding these mistakes, you can confidently convert moles to grams with accuracy and precision.
Applications in Chemistry
Converting moles to grams is fundamental to many areas of chemistry, impacting both theoretical understanding and practical applications. Some key areas where this conversion is applied include:
- Stoichiometry: This field involves calculating the amounts of reactants and products in chemical reactions. Moles-to-grams conversions are essential for determining the mass of substances involved, ensuring reactions are balanced and efficient.
- Molecular weight determination: By converting moles to grams, chemists can determine the molecular weight of unknown substances, aiding in the identification and characterization of compounds.
- Solution preparation: Accurate preparation of solutions requires precise measurement of solute mass. Converting moles to grams ensures the correct concentration is achieved, crucial for reliable experimental results.
- Quantitative analysis: In analytical chemistry, converting moles to grams is used to quantify the concentration of substances in samples, supporting accurate analysis and quality control.
- Industrial applications: In industries such as pharmaceuticals and manufacturing, converting moles to grams is vital for scaling up chemical processes and ensuring product quality and consistency.
These applications underscore the significance of converting moles to grams in advancing chemical research and practical applications.
Why is Converting Moles to Grams Important?
Understanding the importance of converting moles to grams is crucial for both academic and practical pursuits in chemistry. This conversion is significant for several reasons:
- Precision in chemical reactions: Accurate calculations of reactant and product masses ensure that chemical reactions proceed as intended, avoiding waste and optimizing yields.
- Safe handling of chemicals: Converting moles to grams allows for precise measurement of potentially hazardous substances, ensuring safe handling and compliance with safety regulations.
- Facilitating communication: Using grams as a common unit of mass simplifies communication among chemists, enabling clear and consistent reporting of experimental results.
- Enhancing experimental reproducibility: Precise mole-to-gram conversions contribute to reproducibility in research, allowing scientists to reliably replicate and validate findings.
- Supporting educational understanding: Learning to convert moles to grams reinforces fundamental concepts in chemistry, fostering a deeper understanding of the subject and its applications.
By recognizing the importance of this conversion, students and professionals can appreciate its role in advancing chemical knowledge and practice.
How Does Temperature Affect Mole to Gram Conversion?
While temperature does not directly affect the conversion of moles to grams, it can influence the physical properties of substances, impacting their behavior in chemical reactions. Here are a few ways temperature can indirectly affect these conversions:
- State changes: Temperature changes can cause substances to change states (e.g., solid to liquid), affecting their density and volume. This can influence measurements and calculations, although the mass remains constant.
- Reaction rates: Temperature can impact the rate of chemical reactions, influencing the time required to achieve equilibrium. While this does not affect the conversion itself, it may affect the timing of measurements.
- Solubility: The solubility of substances can be temperature-dependent, affecting the concentration of solutions prepared by converting moles to grams.
While temperature does not alter the fundamental relationship between moles and grams, it is essential to consider its effects on experimental conditions and outcomes.
Tools for Converting Moles to Grams
Several tools and resources can facilitate the conversion of moles to grams, enhancing efficiency and accuracy. These include:
- Periodic tables: A reliable periodic table provides atomic masses for elements, essential for calculating molar mass.
- Calculators: Scientific calculators can perform complex calculations quickly, reducing the risk of errors in conversions.
- Online converters: Numerous online tools can automatically convert moles to grams, simplifying the process for students and professionals.
- Software applications: Chemistry software can perform a range of calculations, including mole-to-gram conversions, streamlining data analysis and reporting.
These tools support accurate and efficient conversions, aiding in the execution of experiments and calculations.
Advanced Conversion Techniques
For those looking to further refine their skills in converting moles to grams, several advanced techniques can enhance accuracy and efficiency:
- Dimensional analysis: Using dimensional analysis, a systematic method for converting units, can improve the accuracy of conversions by ensuring consistent units throughout calculations.
- Use of significant figures: Applying significant figures rigorously can enhance precision, reflecting the reliability of measurements and calculations.
- Error analysis: Conducting error analysis can identify potential sources of error in mole-to-gram conversions, supporting the refinement of experimental techniques.
- Calibration of equipment: Regular calibration of analytical balances and other equipment ensures accurate mass measurements, critical for precise conversions.
By mastering these advanced techniques, chemists can achieve greater accuracy and reliability in their conversions, supporting their research and practice.
Frequently Asked Questions
What is the difference between moles and grams?
Moles quantify the amount of substance, while grams measure its mass. Moles are related to the number of particles, whereas grams are a unit of mass.
How do you convert grams to moles?
To convert grams to moles, divide the mass of the substance by its molar mass (in grams per mole).
Why is molar mass important in conversions?
Molar mass provides the conversion factor between moles and grams, allowing for accurate calculations of mass given the amount of substance.
Can temperature affect mole-to-gram conversions?
Temperature does not directly affect the conversion, but it can influence the physical properties and behavior of substances in reactions.
What tools can help with mole-to-gram conversions?
Tools such as periodic tables, calculators, online converters, and chemistry software can facilitate accurate and efficient conversions.
How can I avoid common mistakes in mole-to-gram conversions?
To avoid mistakes, ensure accurate determination of molar mass, consistent units, and appropriate use of significant figures. Verify calculations to prevent errors.
External Link
For further reading on mole-to-gram conversions and related topics, visit Khan Academy's Chemistry Section, a trusted educational resource.
Conclusion
Converting moles to grams is a vital skill in chemistry, underpinning many calculations and applications in the field. By understanding the relationship between moles, grams, and molar mass, chemists can accurately determine the mass of substances involved in reactions. This comprehensive guide has provided the tools and knowledge needed to master this conversion, enhancing both academic and practical pursuits in chemistry. With careful attention to detail and the use of reliable resources, you can confidently perform mole-to-gram conversions, supporting your work and studies in the scientific community.